Version 3.0 (August 1, 2003)
-
Inverse PCR
1. Sau3A I Digestion
Prepare for Sau 3A I Mixture in a 6.5 ml tube.
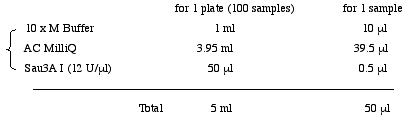

<Biomek; #1 Digestion> Add 50µl Sau3AI mixture to 50µl of genomic DNA in 96-well round bottom plate( IWAKI).

Seal the plate with TAPE 48 (Nunc) and close the lid. Seal the lid with vinyl tape around the plate.

Incubate at 37oC for 3h.

Incubate at 65oC for 1h (BM Synthetic Oven).
2. Ligation
Prepare Ligase Mixture in a 15ml tube.
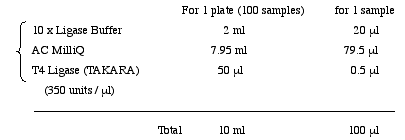

Pour Ligase Mixture into 1/4 reservoir.

<Biomek; #2 Ligation> Add 100µl Ligase Mixture to 100µl digested DNA. To dispense the mixture into the final row, transfer Ligase Mixture in reservoir to 6.5 ml Assist Tube.

Seal the plate with TAPE 48 (Nunc) and close the lid. Wrap PARAFILM around the plate.

Incubate at 4 oC overnight
3. MultiScreen-PCR (MILLIPORE)
<Biomek; #3 NU plate> Load ligated DNA into the MultiScreen-PCR plate on top of the MultiScreen manifold. Apply vacuum at 15 inches Hg for 13minutes. Add 30µl TE to each well.

Shake the MultiScreen plate vigorously on a plate mixer for 5 minutes.

Retrieve purified DNA from each well with a multichannel pipettor.
4. PCR
Prepare PCR Mixture in a 6.5 ml tube.
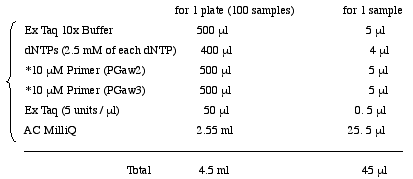

<Biomek; #4 PCR> Load 45µl PCR Mixture into MicroAmp Optical 96-well Reaction Plate(PE). Add 5µl purified DNA.

Put the Full Plate Cover on the plate. Place the plate in PE9700.
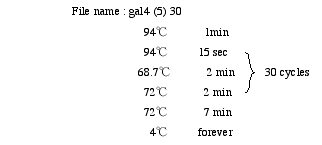
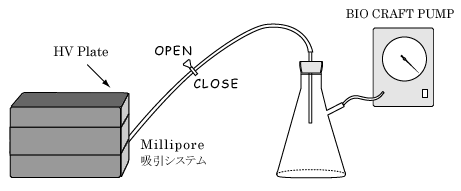
5. MultiScreen (Sephacryl S-400)
S400:FWashed and autoclaved. Stored at RT as a 1:1 mixture.
Add 3 µl of 0.04% BPB/ TSE to PCR product. Mix and spin down.
Put HV plate on top of the MultiScreen manifold.

<To pack the column> Vigorously shake the bottle of S400 to make an even slurry and pour in a reservoir (eppendorf). Load 125µl of well-mixed S400 into each well of the HV plate (MILLIPORE) with multichannel pipettor (eppendorf).

Apply vacuum until dry.

Repeat adding S-400 and vacuum(S400:F0.625 ml/well)4 more times.

<Wash> Load 50µl of TSE into each well with multichannel pipettor (eppendorf).

Apply vacuum until dry.

Repeat adding TSE and vacuum(TSE:F100 µl/well)
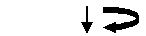
Centrifuge the HV plate on a reservoir
2400 rpm, 3min, 25oC
Place the HV plate on a fresh round bottom plate for collection. Apply 25µl of DNA sample to the top of the gel.
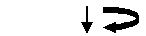
2400 rpm, 3min, 25oC
Use eluted DNA as template of sequence reaction.
6. Sequence Reaction
Prepare BDT mixture in a 1.5 ml tube.

<Biomek; #6 Sequence Reaction> Add 7µl of BDT mixture to the MicroAmp Optical 96-well Reaction Plate(PE). Then add ‚3µl of template purified by S400 column.

Put the Full Plate Cover on the plate. Place the plate in PE9700.

7. EtOH precipitation
<Biomek; #7 EtOH Precipitation> Add 8 µl of AC MilliQ and 32 µl of 100% EtOH (RT) to each well of the plate that was finished Sequence reaction.

Incubate for 15 min at RT.
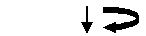
6000 rpm, 15 min, 25oC
Without disturbing the precipitates, discard the supernatant by inverting the plate onto Kimtowels.

Rinse the pellet by adding 75 µl of 70% EtOH to each well.
6000 rpm, 15 min, 25oC
Remove the supernatant as above. Leave the plate at 37oC covered with aluminum foil to dry up precipitate.
8. Sequencer
Add 30 µl of ACMilliQ to the plate.

Seal the plate with adhesive tape(Nunc), voltex, and spin down.

Remove the half volume (15 µl) of each sample to a new round bottom plate. Seal the plate with Easy Peel and store at -80oC.

Seal the remaining plate (MicroAmp Optical 96-well Reaction Plate(PE)) with adhesive-backed aluminum foil tape.

Set the plate in ABI3700.
* Primer
4. PCR
PGaw2
Sequence (5' to 3') : CAG ATA GAT TGG CTT CAG TGG AGA CTG
DeprotectedPGaw3
Sequence (5' to 3') : CGC ATG CTT GTT CGA TAG AAG AC
Deprotected6. Sequence Reaction
Sp1
Sequence (5' to 3') : ACA CAA CCT TTC CTC TCA ACA A
Unpurified, Trityl Off
- Inverse
PCR-3'
1. Picking
<Biomek Picking Temp>
Pick up 5µl each of necessary samples. Transfer DNA after ligation and purification by MultiScreen PCR to a fresh MicroAmp Optical 96-well Reaction Plate (PE) .2. PCR
Prepare PCR Mixture in 6.5 ml tube.
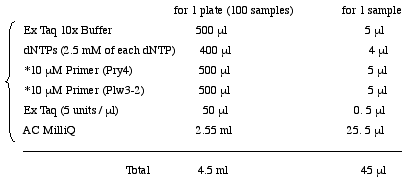

<Biomek; #4 PCR> Add 45 µl of PCR mixture to the plate 1.

Put the Full Plate Cover on the plate. Place the plate in PE9700.

*Primer
Pry4 (5' to 3') CAA TCA TAT CGC TGT CTC ACT CA(Unpurified, Trithyl Off)
Plw3-2 (5' to 3') TAA CCC TTA GCA TGT CCG TGG GGT TTG(Unpurified, Trithyl Off)
3. Nested PCR
Prepare PCR Mixture in 6.5 ml tube.
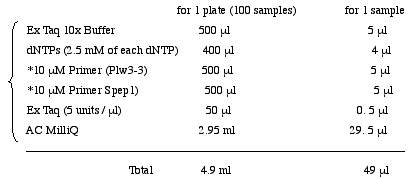

<Biomek; Nested PCR> Load 49µl PCR Mixture into MicroAmp Optical 96-well Reaction Plate(PE). Add 5µl of first PCR product.

Put the Full Plate Cover on the plate. Place the plate in PE9700.

*Primer
Plw3-3 (5' to 3') CAA AGC TCT AGC TAG AGG ATC(Unpurified, Trithyl Off)
Spep1 (5' to 3') GAC ACT CAG AAT ACT ATT C(Unpurified, Trithyl Off)4. MultiScreen (Sephacryl S-400)
5. Sequence Reaction
Primer: Spep1 (5' to 3') GAC ACT CAG AAT ACT ATT C
6. EtOH precipitation
7. Sequencer
This database is based on the DYNACLUST system developed by DYNACOM Co., Ltd.